Esterification - Formation of aroma/ester
Esterification - Formation of aroma/ester
An ester is a chemical compound derived from an acid (organic or inorganic) in which at least one –OH hydroxyl group is replaced by an –O– alkyl (alkoxy) group, as in the substitution reaction of a carboxylic acid and an alcohol.
RCO2H + R′OH ⇌ RCO2R′ + H2O
Types of esters
There are two groups of esters, aliphatic and phenolic. Aliphatic esters are those formed with straight chain/non-cyclic molecules (such as alcohols and fatty acids). Phenolic esters are formed from phenolic compounds, which are cyclic in nature.
Example:
Ethanoic acid (Common Name: Acetic acid) CH3COOH
Ethanol C2 H5OH
CH3COOH + C2 H5OH ⇌ CH3COOC2 H5 + H2O
In the presence of a dehydrating agent, e.g. H2SO4, the reaction goes to the right-hand side of the equation:
CH3COOH + C2 H5OH ---> CH3COOC2 H5 + H2O
It is said that aliphatic monocarboxylic esters are the most significant esters for white wines. The second group are those formed from acetic acid and higher alcohols.
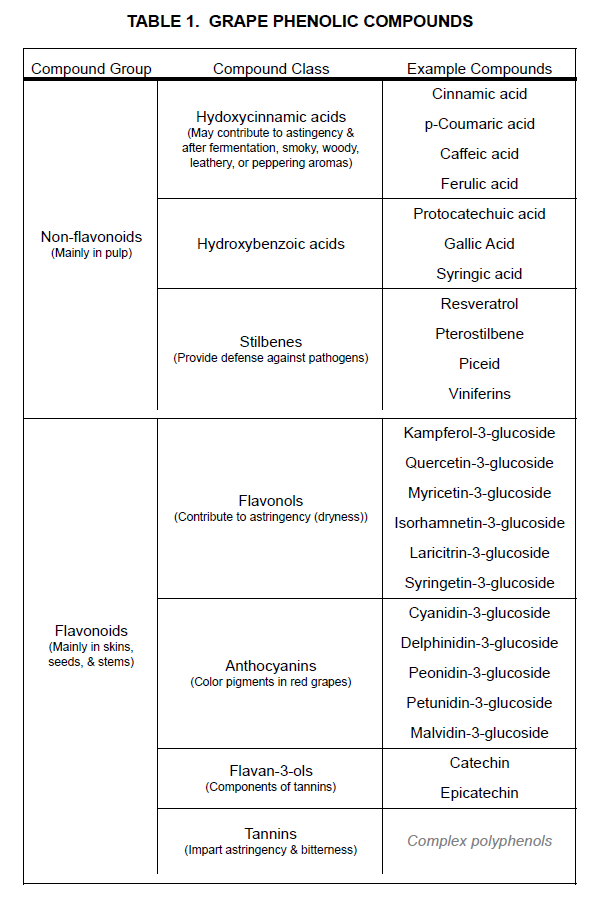
Example: Grape phenolic compounds
Further reading:
1. Blogs wirtten by some wine advocate(s): https://enoviti-hanumangirl.blogspot.com/2013_02_01_archive.html





תגובות
הוספת תגובה